Defense Minister Benny Gantz announced on Thursday that the Israel Institute for Biological Research in Ness Ziona will begin human trials on a vaccine for COVID-19 after the Jewish holidays in September.
“The successful preliminary trials raise much hope,” said Gantz. “The next step, as agreed, is the beginning of human trials after the High Holidays. This will be done in coordination with the Health Ministry and according to all medical safety requirements.”
Prof. Shmuel Shapira, director of the institute, was optimistic about the prospects of a vaccine.
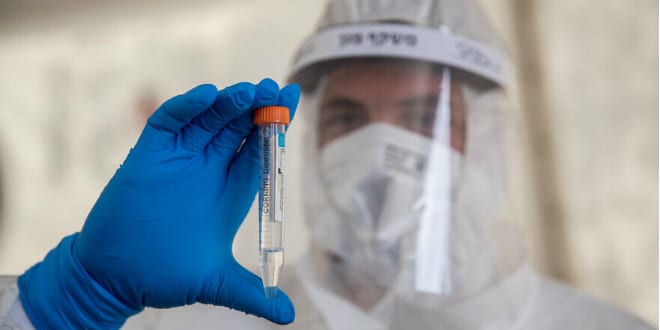
“We have a terrific vaccine,” said Shapira holding up a vial. “There are regulatory processes that the vaccine needs to go through in order to meet the timetable you laid out. We are starting after the Tishrei holidays with safety and efficiency tests, but we have the product in our hands,”